Bio-inspired Materials: Structural Color in Nature
This post is the first in a series, Bio-inspired Materials: From Nature to Technology.
Nature has developed a material to meet any need. In this series, we explore the science behind natural materials and how researchers use nature’s tricks to design new bio-inspired materials.

Spiderweb photo by Josef F. Stuefer, CC by 2.0
Vivid colors appear throughout nature. Plants and animals, on land and underwater, use color in ways that are both beautiful and practical. Understanding how this color is produced can guide the development of new technologies including anti-reflective coatings and medical devices. Here, I discuss how color is produced. I then present the Papilio blumei butterfly as an example of natural color and show a surprising connection between this beautiful butterfly and the common moth. Finally, I describe new technologies inspired by nature’s use of color and light.
I. Origin of color
Everything that we see is because of light. For you to see an object, light must travel from that object to your eye. For example, consider a stop sign on a sunny day. Light that comes from the sun is a mixture of light rays of all colors—these rays are called photons. When many photons of different colors hit our eye, our brain interprets the combination of colors as white. But, when the light from the sun hits the stop sign, most colors are absorbed, and red light is reflected. Therefore, the light coming from the stop sign that hits our eyes is mostly red light, and the sign appears red. The same principle is true for most things in our everyday life: blueberries reflect blue light and absorb every other color; broccoli reflects green light and absorbs every other color. Most color that we see comes from light of all colors hitting an object, certain colors being reflected, and the rest absorbed. (As an aside, other sources of color include fluorescence, which involves a change in color of light hitting an object, and excitation, such as in a neon light.)
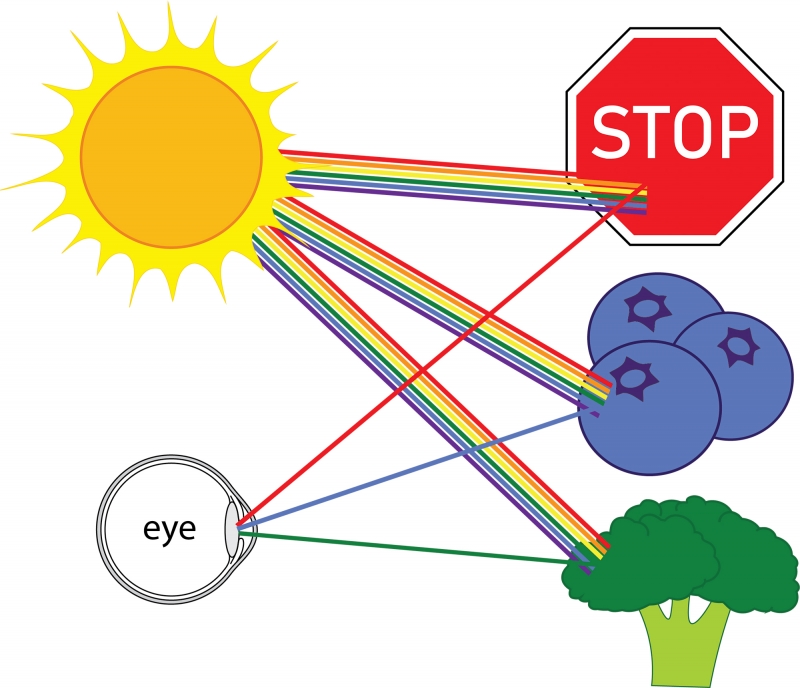
White light hitting various objects, light of specific colors being reflected.
But what determines which colors are reflected? Colors are created in two major ways. The first is familiar to us: the molecules that make up a material absorb some colors of light and reflect others. Examples of these materials include dyes and pigments. The selective absorption occurs because the molecules in the material interact with light of different colors in different ways. One example is chlorophyll, a pigment that gives plants their green color. When white light (a mixture of light of all colors) hits a plant, the chlorophyll absorbs almost all the colors of light (red, blue, etc.). Green, however, is not absorbed. Instead, green light reflects and reaches your eye, and the plant looks green. The fact that only green light is reflected while other colors are absorbed results from the arrangement of atoms within each chlorophyll molecule. Other pigments of different colors have different arrangements of atoms, and therefore reflect different colors of light.
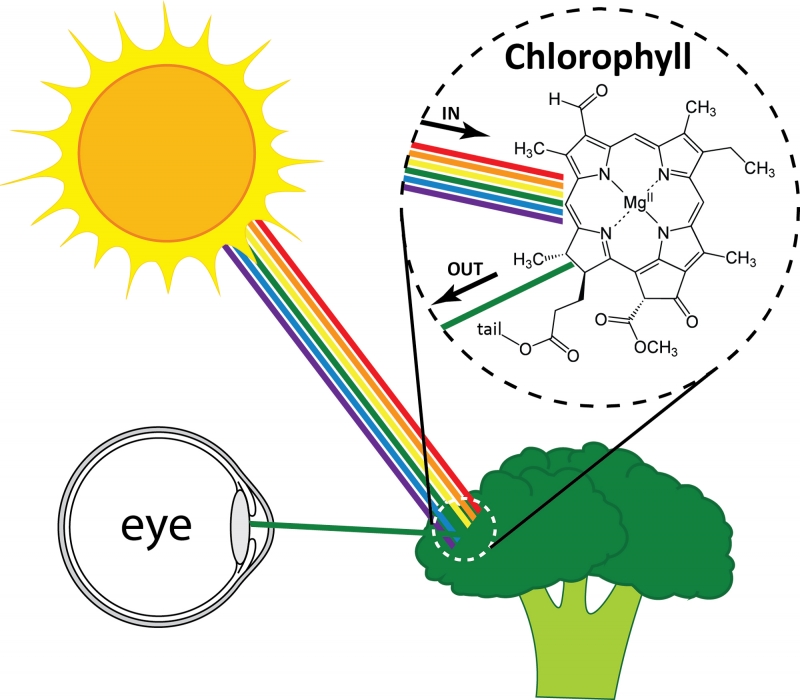
White light hitting broccoli and green light reflecting. The pigment chlorophyll within the broccoli absorbs all colors except green.
Color due to molecular absorption is the most common source of color. Pigments and dyes are particularly vivid examples, but most everyday materials are colored for the same reason: the molecules within the material absorb and reflect different colors of light. Differences in the amount of absorption and reflection also cause blacktop to heat up more than the sidewalk on a sunny day. When light from the sun hits the sidewalk, most of the light is reflected. Since the sidewalk reflects light of most colors, it appears to be an off-white. In contrast, when light from the sun hits the blacktop, most of the light is absorbed, and the material appears black. When light is absorbed, it is converted into heat. Since the blacktop absorbs more light than the sidewalk, it reaches a much higher temperature.
A second way color is produced is called structural color. This form of color is less common but gives some of the most vivid colors in nature. Unlike pigments, which produce color by absorption of specific wavelengths of light, structural color involves reflection of light within a material. If the material contains specific shapes or patterns on the surface, it will reflect specific colors of light. Common examples of structural color are the colors that appear on the surface of a soap bubble or oil slick. The bubble and slick are not intrinsically colorful—the color does not come from a pigment. Instead, the color is produced due to the shape of the material that controls how light reflects inside and ultimately determines what color emerges and reaches your eye. For example, the bubble consists of a thin film of water. White light hits the film, and certain colors reflect and reach your eye. Similarly, an oil slick is a thin film of oil on the surface of water. White light hits the oil layer, and certain colors reflect. The thickness of the bubble or slick determines the colors that reflect. At different locations on the bubble or slick, the thicknesses of the water or oil layers are slightly different, and so the bubble or slick appear to be different colors in different regions. To understand why different colors reflect, we must consider the nature of light.
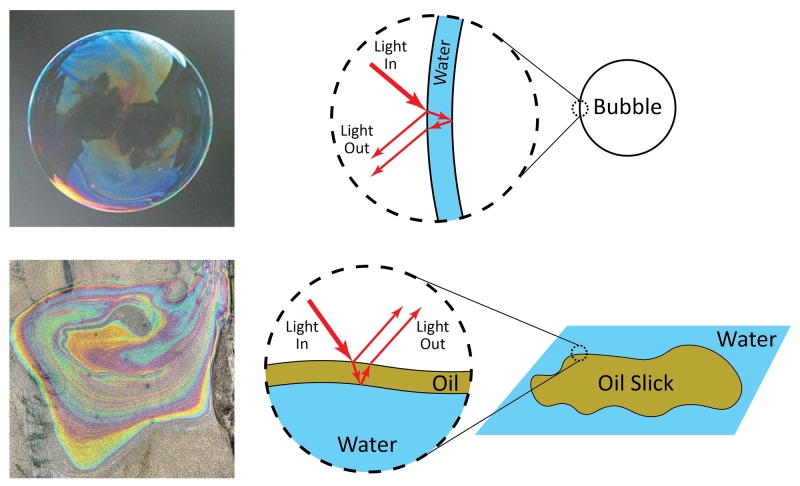
Soap bubbles and oil slicks show structural color. Bubble photo by Rhett Maxwell, CC by 2.0. Oil slick photo by Gary Robinson
Structural color exists because light travels as waves. Light waves are similar in many ways to water waves. For example, ripples on the surface of a pond have a certain spacing, called a wavelength, and travel at a certain speed. Light waves behave like these ripples, but the wavelength of a light wave is much smaller, and the speed of light is much faster. One important property of waves is the ability to combine with other waves. When the crests of two waves come together, they add, resulting in a crest that is twice as high. Conversely, if the crest of one wave meets the trough of another wave, they cancel each other out, momentarily leaving smooth water. Just like water waves, light waves combine with each other and can either add together or cancel each other out. In the case of the oil slick or soap bubble, light of all colors hits the thin film of water in the bubble or thin film of oil in the slick. As shown in the figure above, some of the light reflects off the top surface of the film, and some light enters the film. The light that enters then reflects off the inner surface of the film. The light waves from these two reflections then combine as they leave the film. Some wavelengths of light add to each other and grow, and some cancel each other out. Only the wavelengths that add together and grow reach your eye—these are the colors that you see.
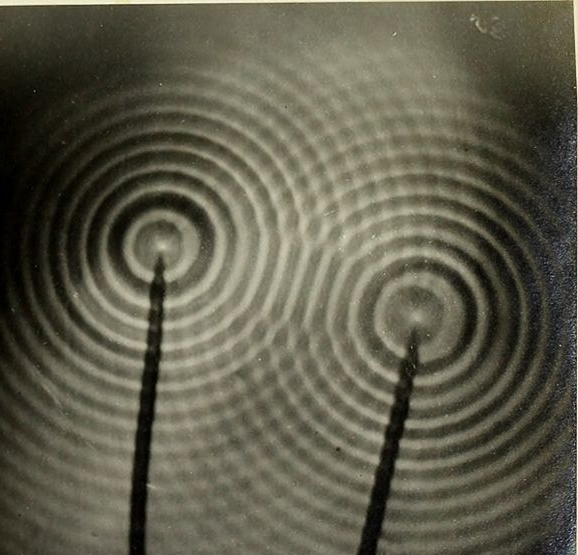
Two sets of waves encountering each other form a pattern of peaks and troughs.
II. Bright butterflies, dull moths
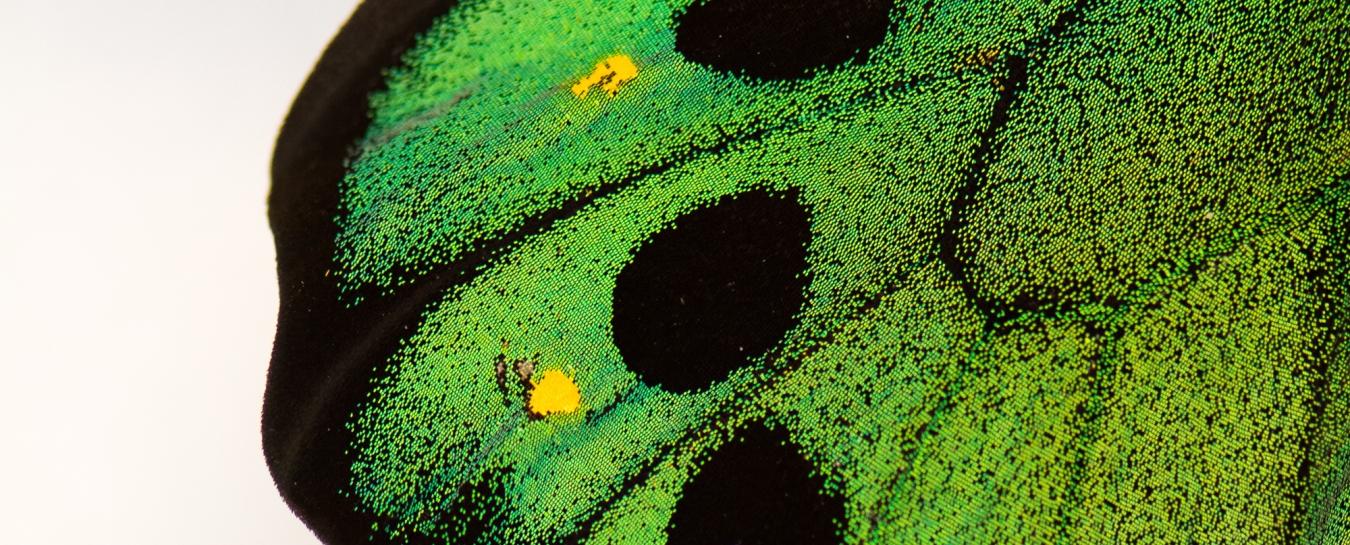
Another source of structural color is microscopic patterns on the surface of materials. This type of structural color is responsible for the coloration of some species of butterflies, beetles, and birds, including the Papilio blumei butterfly. Microscope images of the wings of this butterfly reveal intricate structures on the surface of the wings. It is these structures that give the butterfly wings their blue and green color. Just like the oil slick or soap bubble, the butterfly wings are not colored by a pigment—the color results from reflection of certain colors of light. Patterns on the surfaces of the scales determine the color, and in the case of the Papilio blumei the patterns reflect blue or green light. The colors of the bubble, slick, and butterfly result from the same physical principle of wave interference. However, since the scales are solid materials rather than liquid like the bubble or slick, the color is permanent. One interesting feature of the butterfly color is that the color changes from blue to green depending on what angle you look at it. This occurs because when light hits the scales at an angle, the path of reflections that the light follows is different than when light hits the scales straight on. The differences in the reflection paths cause different colors to emerge and reach your eye.
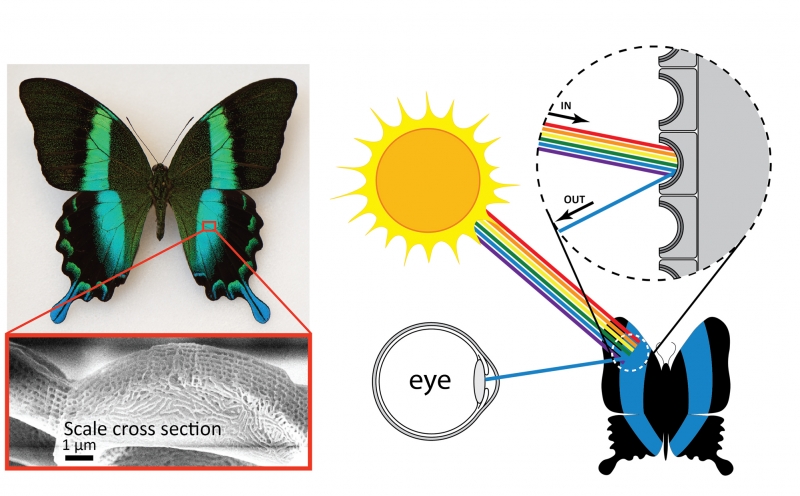
Microscopic structures on the surface of the scales of the Papilio blumei butterfly give the butterfly its striking blue-green color. Wing scale image from Tam et al. “Iridescence and nano-structure differences in Papilio butterflies,” Optical Materials Express (2013). Reproduced with permission from The Optical Society.
Surface structures can have other uses besides creating color, however. A moth uses structures on the surface of its eye to prevent reflection and maximize the amount of light that enters the eye. This allows the moth to see in low-light conditions and avoid predators by reducing light reflecting from the eye. In this way, the moth uses structures to achieve the opposite effect of the butterfly: preventing reflection rather than reflecting specific colors.
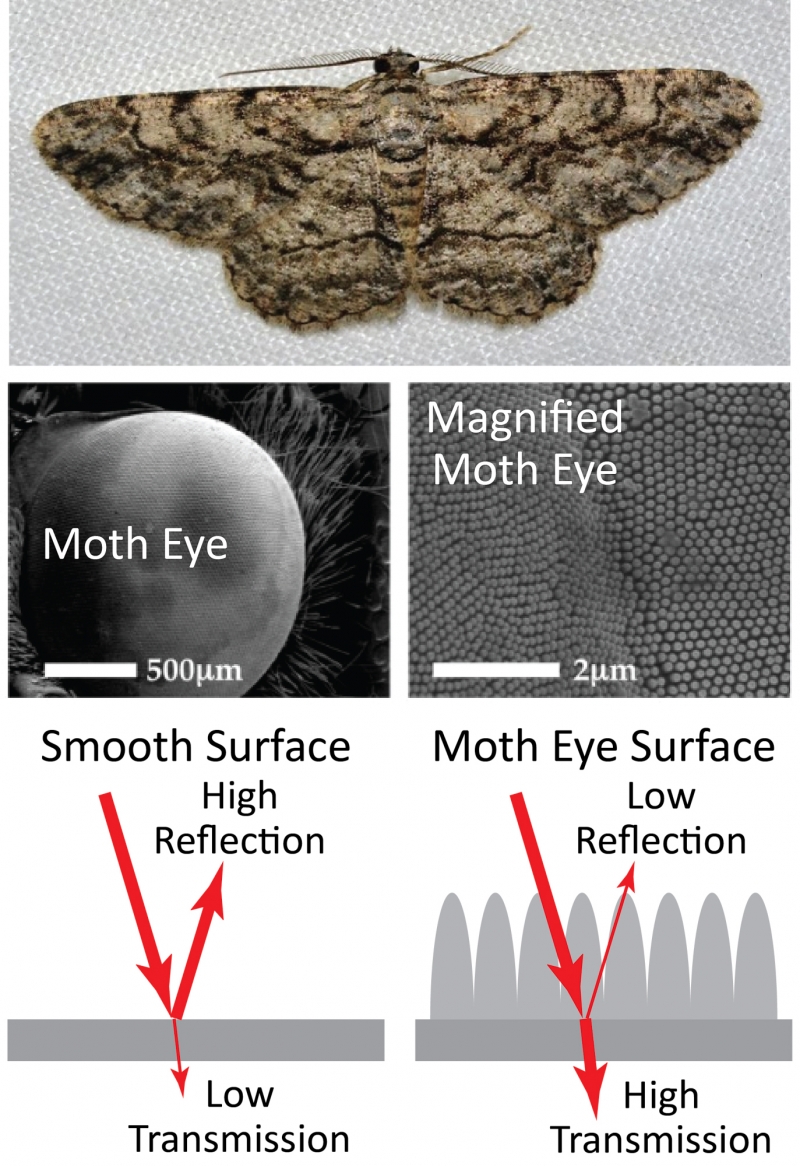
Top: common gray moth. Middle: microscope images showing two different magnifications of the moth eye. The second of these images shows the microscopic structures on the surface of the eye. The structures prevent reflection and allows the moth to see in low-light conditions. Bottom: diagram illustrating how the structures on the surface reduce reflections by increasing the transmission of light through the surface. Moth photo by Andy Reago and Chrissy McClarren, CC by 2.0. Moth eye images from Gonzalez et. al, "Bio-inspired, sub-wavelength surface structures for ultra-broadband, omni-directional anti-reflection in the mid and far IR," Optics Express (2014). Reproduced with permission from The Optical Society.
III. Bioinspiration and technology development
Mimicking nature has led to the development of new technologies for many different applications. Researchers at the University of California, Santa Barbara developed a technique for making anti-reflective surfaces inspired by the moth eye. To do this, they used a technique called colloidal lithography. In this technique, an initially smooth surface is etched into the patterns seen in the figure below. Similar surface preparation techniques can be used to mimic the patterns of the butterfly wing, yielding surfaces of different colors with potential applications for vivid displays and colorful coatings.
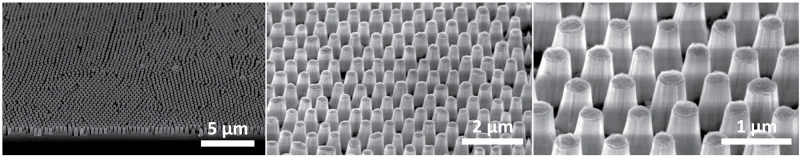
Anti-reflective surfaces inspired by the moth eye. Figure shows increasing magnification of the patterns responsible for preventing reflection. Microscope images by Dr. Lesley Chan.
Another application of structural color is medical devices. For example, the figure below shows a layered material that changes color as you stretch it. This material has been proposed for use in bandages as an indicator for swelling. Like the other technologies presented above, this material was inspired by nature. The bandages mimic the layered structure of the fruit of the Margaritaria nobilis plant, which uses layers to create an iridescent blue color.
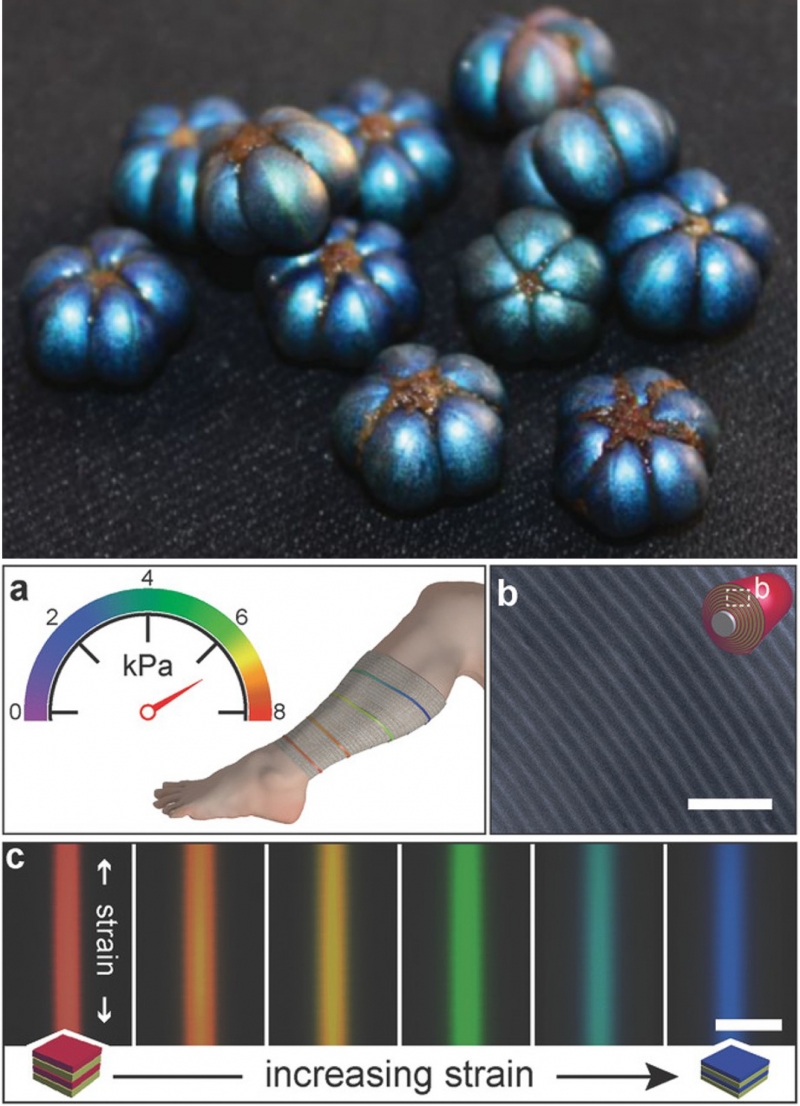
Top: the iridescent blue color of the fruit of Margaritaria nobilis results from its layered structure. Bottom: Color-changing materials for improved bandages. The color of the bandages results from a layered structure inspired by Margaritaria nobilis. As the materials is stretched, the layer thicknesses change, resulting in a change of color. Margaritaria nobilis image from Vignolini et al. “Structural colour from helicoidal cell-wall architecture in fruits of Margaritaria nobilis.” Journal of The Royal Society Interface 2016, CC by 4.0. Bandage figure from Sandt et al. “Stretchable Optomechanical Fiber Sensors for Pressure Determination in Compressive Medical Textiles,” Advanced Healthcare Materials (2018). Reproduced with permission from John Wiley and Sons.
There are many more examples of structural color in nature, and many more sources of inspiration for the development of new technology. Full realization of the potential of bio-inspired technologies will require the collaborative efforts of biologists, physicists, and engineers, all seeking to understand and mimic the amazing natural materials around us.
About the author
George Degen is a doctoral student in chemical engineering at University of California, Santa Barbara. His research focuses on understanding marine mussel adhesion to guide the design of improved wet adhesives for biomedical applications. He first became involved with the Museum in March 2020 when he presented his research during Science Pub: Sticky When Wet—Underwater Superglue from Marine Mussels.





3 Comments
Post a CommentFascinating content! The author did a really good explaining the material!!
Super awesome, knowing how it works just made me admire and wonder the nature. Great Author!!
Beautifully described. Thank you for the information.